Aluminum dross is far from worthless—especially when it contains significant amounts of recoverable metallic aluminum. But getting that metal out efficiently and cleanly is both a technical and economic challenge.
In this article, we break down how aluminum is recovered from dross, what factors affect recovery rates, and which separation techniques are most effective.
Why Is Recovery Challenging?
- Metallic aluminum in dross exists in various forms:
- Coarse metal chunks embedded in oxide
- Fine droplets or globules trapped in porous matrix
- Bound compounds (e.g., aluminum nitride, spinels)
The challenge is to extract as much free aluminum as possible, without excessive oxidation, contamination, or energy loss.
Key Recovery Methods
Here are the primary strategies for recovering metallic aluminum from dross:
Mechanical Separation
Used mainly for white or high-metal dross, this method focuses on liberating and sorting metal particles.
Techniques include:
- Crushing & screening
- Density separation (metal sinks, oxides float)
- Magnetic or eddy current separation (for non-ferrous sorting)
Advantages:
- Low energy use
- Good for coarse aluminum fractions
- Scalable and simple
Limitations:
- Ineffective for fine metallic particles
- Cannot recover chemically bound aluminum
Thermal Recovery (Melting)
This is the most direct and effective method to recover metallic aluminum, particularly from black dross and fine fractions.
Common approaches:
- Rotary Furnaces: Add salt flux to reduce oxidation and facilitate metal separation
- Tilting rotary furnaces: More efficient, allow metal skimming
- Plasma or induction melting: Emerging for high-purity or salt-free processing
Advantages:
- High recovery rates (up to 90% with optimized control)
- Can treat fine and complex dross
Limitations:
- Generates salt slag, which requires further treatment
- Higher energy input
- Emission controls required
Chemical Extraction (Hydrometallurgy)
While not suited for recovering metallic aluminum directly, hydrometallurgical processes can:
- Extract aluminum compounds from dross or salt slag
- Neutralize aluminum nitride (AlN), which reacts violently with water
Mainly used for residue treatment, not metal recovery.
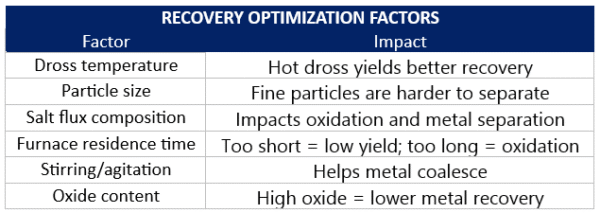
Recovery Optimization Factors
Several variables determine how much aluminum you can recover from a given dross batch:
- Dross temperatura → Hot dross yields better recovery
- Particle size → Fine particles are harder to separate
- Salt flux composition → Impacts oxidation and metal separation
- Furnace residence time → Too short = low yield; too long = oxidation
- Stirring/agitation → Helps metal coalesce
- Oxide content → High oxide = lower metal recovery
Typical Recovery Rates
Advanced operators may use two-stage recovery: mechanical + thermal, followed by residue treatment.
What Happens After Recovery?
After metallic aluminum is recovered, it can:
- Be re-melted and cast into ingots
- Enter alloy production as a secondary input
- Undergo refining if purity is low
Meanwhile, the non-metallic residues (salts, oxides) are either:
- Processed for reuse (e.g., in cement or ceramic fillers)
- Treated to meet environmental discharge standards
Summary
Recovering metallic aluminum from dross is not just about material value—it’s about:
- Resource efficiency
- Environmental compliance
- Sustainable production economics
The key is to optimize separation and melting while minimizing secondary waste and energy use.
Does this topic interest you? Discover our thematic dossier:
→ Aluminium dross recycling: overview and key technical challenges

